CROs Moving Away from Manual Systems and Searching for the Best CTMS

CROs are moving away from manual systems and looking for unified clinical model as a software solution. The necessity for this is forced by the contemporary approach of end-to-end systems, as well as the need of seamless collaboration between all internal and external stakeholders. In addition, clinical trial software solutions deliver better metrics-driven insights across the lifecycle thus enhancing performance. In fact, almost 100% of CROs express the need to unify their current clinical trial applications, such as CTMS, EDC, and eTMF. The reason that underlies is the priority to speed study completion, save costs and gain better study quality and visibility.
CROs also report that one of the greatest challenges they have is the integration between the CTMS and EDC. Basically, organizations are striving to implement a system, which includes both, otherwise it wouldn’t have the ability to improve clinical operations. Despite the challenges, CROs are looking to move away from manual systems and implement new softwares. Statistics say that more than a 40% have already implemented an eTMF application. In comparison to 2014 this percentage has been doubled. Again, during that same year, there were nearly 50 % of the organizations that still used paper based documents. Now, the CROs doing this have dropped to 10 %.
Interestingly enough, CROs are more into utilizing new solutions than sponsors. In general, CROs want to improve their study processes, achieve significant benefits and get better visibility to performance metrics as well as go a step further into automated tracking and reporting of documents. CROs also state the need to unify their clinical applications, due to faster study execution, cost savings and improved study quality. What drives such unification? On the average, the top CROs manage about 50% of the $25 billion clinical CRO market in terms of revenue. This increases the necessity for tools that bring about better visibility and insights across the clinical trial landscape.
Which are the most commonly used applications?
Reported by the majority of CROs, these include EDC (86%), eTMF (62%), and safety systems (60%). Where is the CTMS positioned? Surprisingly, it is still beaten by spreadsheets when it comes to the study and sites management. To be more precise, spreadsheets are used by 72% of CROs, while only 48% use a CTMS application and 64% use eTMF. The problem is that the predominant use of spreadsheets will contribute to the collaboration challenges reported by both CROs and sponsors.
What is the impact of CTMS when it comes to clinical operations?
CTMS is undoubtedly important for CROs and life sciences organizations. It is estimated that in 3 years, there will be an increase of CTMS implementation by 15%. The drive underneath is the rising demand for data and site collection softwares, as well as the CTMS market growth and the appearance of new and more contemporary applications.
Of course, many of the CROs report major problems with their current CTMS solutions, such as the inability for integration with EDC (50%), not user-friendly interface (50%), hardships in tracking and reporting (40%). On the side of the sponsors, they confirm that tracking and reporting (40%) and ease of use (35%) seem to be the top challenges.
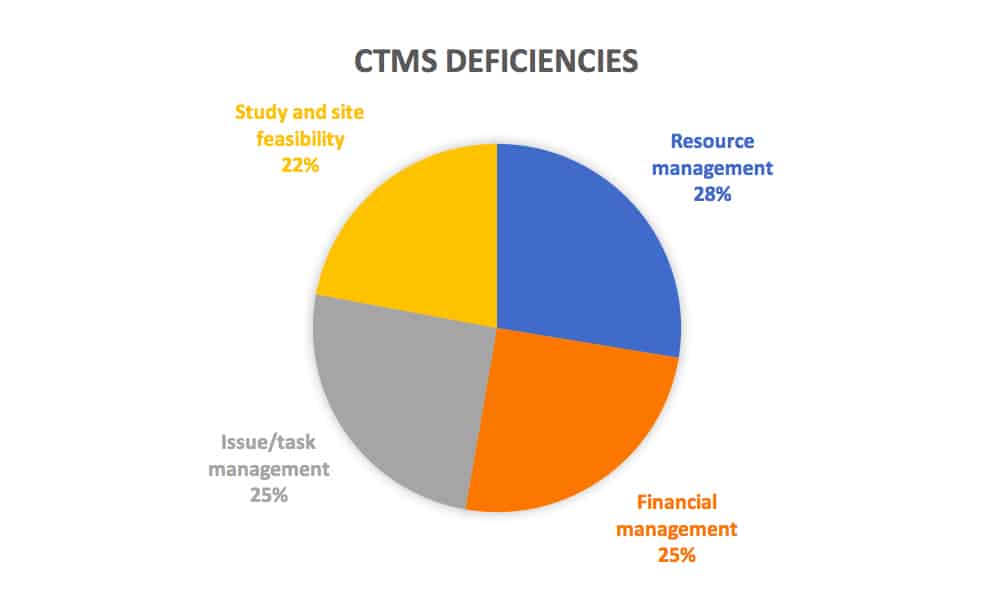
In addition, CROs also report CTMS deficiencies like the inability to fully support key functions like resource management (80%), financial management (73%), issue/task management (66%), and study and site feasibility (64%).
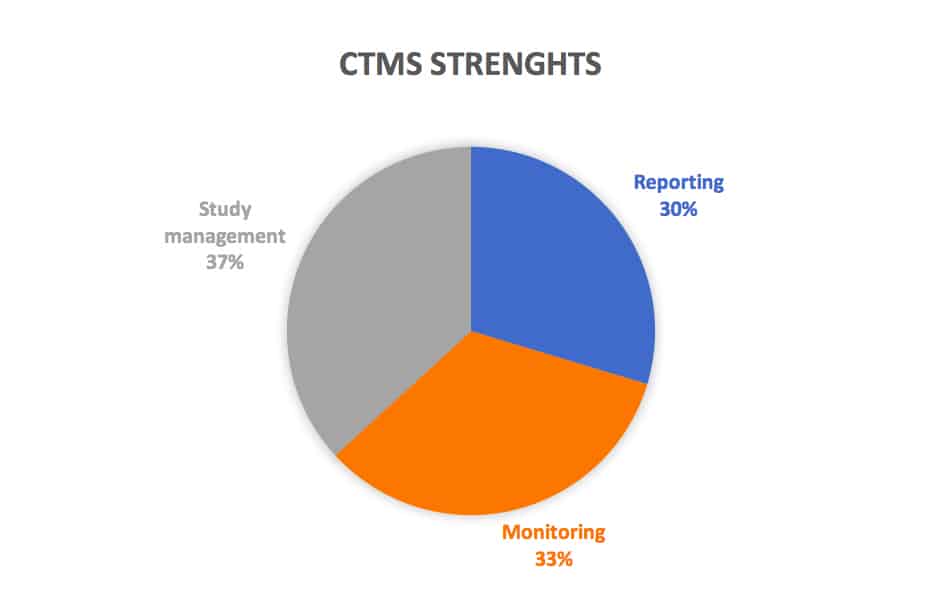
Despite the cons, CROs also report benefits like the reporting site management functions (54%), monitoring (61%), and study management (67%) as fully supported by their CTMS applications.
Since sponsors outsource more clinical trial management activities to CROs, the latter are being heavier users of CTMS application. Due to this fact, the CROs’ need for CTMS/EDC integration is deeper than sponsors, which results in lesser sponsor’ need for an in-house CTMS. With trials becoming increasingly complex and specialized, there is an industrywide recognition that unifying clinical processes and systems. This is a must if one wants to bring about quality and speed of study execution. The top challenge seems to be the integration of multiple applications within the clinical solutions. Still, many of the first systems used in the past, but continuously in function today, lack the functionality necessary for true end-to-end processes, visibility, and collaboration. All CROs report their old CTMS softwares only partially support key clinical processes, which results in the need for manual tracking and reporting. Therefore, CROs are increasingly looking for a contemporary, user-friendly, unified clinical solution to tackle these challenges and enable faster, higher quality studies, in order to remain competitive.
Clinical trial outsourcing is predicted to exceed 70% by 2020. Increasing study execution and quality, and at the same time the increasing complexity across the clinical lifecycle, signify the need for a unified clinical model that is characterized by end-to-end processes and systems, seamless collaboration across the clinical ecosystem, as well as greater insights from metrics to increase performance.
This is why Clinicubes is the best software solution out there. The CTMS we have developed is modern looking, user-friendly and will include all functions you need, and of course can be easily integrated with other solutions you are currently using. The trick we have is that the CTMS is purpose-built and will adapt to your organizations’ needs and not the other way around. Born in the Cloud, Clinicubes CTMS will help you increase visit reporting by up to 50%, while at the same time will significantly reduce operational costs.
You can download the PDF version of this article here: CROs in Need of CTMS
Report & statistics source: Veeva




