Clinicubes product features
The CTMS that offers numerous benefits and integrated solutions for every aspect and phase of the clinical studies
Management
Site Tracking
Database
Tools
Patient
randomization
randomization
24/7 Support
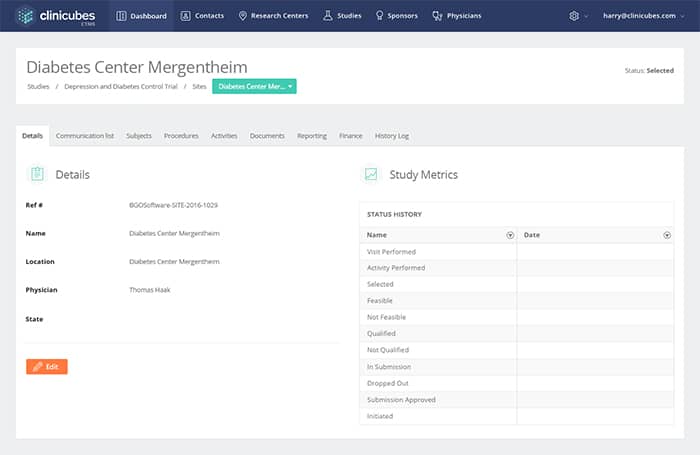
Clinical Trials Management
Effective management of clinical trials
- Instant preview of timetables, financial information, objectives
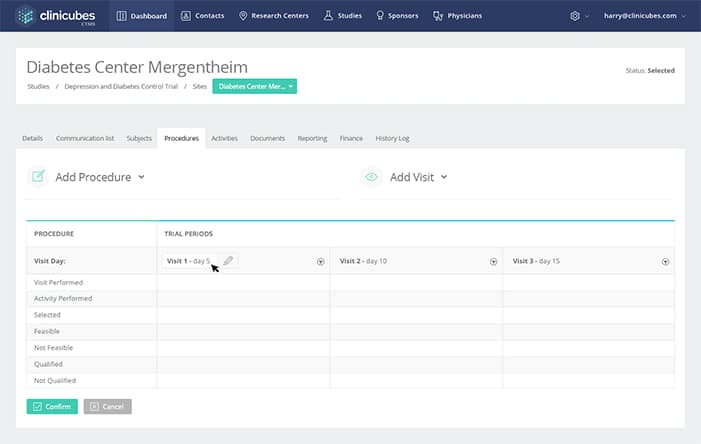
- Quick monitoring of study details
- Management of procedures and activities
- Overview of study budget and financial forecasting
- Management and tracking of trial information
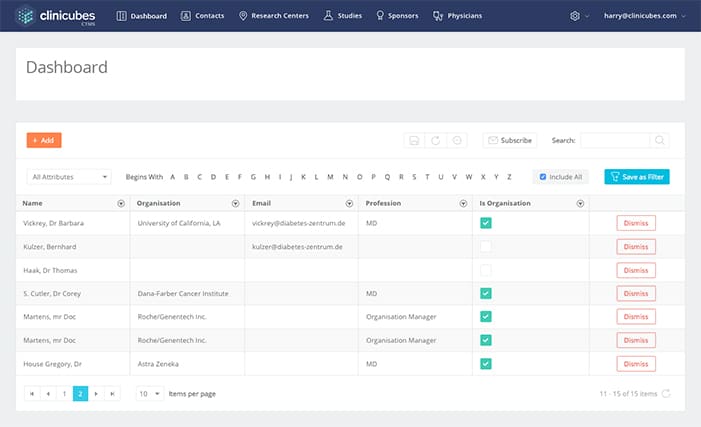
Site Tracking & Management
Enables reliable control over sites and subject database
- Site management and regulatory process tracking
- Subjects database and study enrollment tracking
Physician’s & Health Service Institutions Database
Drives clinical trial performance in a smooth way
- Physician’s and health service institutions database systemization
- Collection and analysis of the studies from different locations
- Communication of study performance data trough interactive dashboards
Project Tracking & Collaboration Tools
Enhances communication and brings about excellence in the working processes
- Easy collection and tracking of various documents
- Actual and projected financial information on the dashboard
- Effective reporting
- Scheduling tools and associated calendar
Patient
randomization
Adaptable randomization for all types of
randomized clinical trials
- Various randomization methods, adaptable to different scenarios and population sample size
- Covariate adaptive randomization model independent
of the expected number of subjects - Setup and guidance by experienced statisticians
Integration & Support
Shapes results and unifies all research technologies used
- Integration with most popular clinical research applications
- Migration and import of data collected with legacy tools
- Superior 24/7 support and ad hoc training